
Exhibit 99.1

ENSYSCE BIOSCIENCES INVESTOR PRESENTATION FEBRUARY 2021

General This presentation (the “ Presentation ”) has been prepared to assist interested parties in making their own evaluation with respect to a potential business combina tio n between Leisure Acquisition Corp. (“ Leisure ”) and Ensysce Biosciences, Inc. (“ Ensysce ”) and the related transactions (the “ Proposed Business Combination ”) and for no other purpose. Neither the Securities and Exchange Commission (the “ SEC ”) nor any securities commission of any other U.S. or non - U.S. jurisdiction has approved or disapproved of the Proposed Business Combination presented herein, or determined that this Prese nta tion is truthful or complete. Any representation to the contrary is a criminal offense. No representations or warranties, express or implied, are given in, or in respect of, this Presentation. To the fullest exten t p ermitted by law, in no circumstances will Leisure, Ensysce or any of their respective subsidiaries, stockholders, affiliates, representatives, directors, officers, employees, advisers, or agents be responsible or liable for a direct, indir ect , or consequential loss or loss of profit arising from the use of this Presentation, its content, its omissions, reliance on the information contained within it, or on opinions communicated in relation thereto or otherwise arising in connection there wit h. Industry and market data used in this Presentation have been obtained from third - party industry publications and sources as well as from research reports prepared for other purposes. Neither Leisure nor Ensysce has indepe nde ntly verified the data obtained from these sources and cannot assure you of the data’s accuracy or completeness. This data is subject to change. In addition, this Presentation does not purport to be all - inclusive or to contain all of the information that may be required to make a full analysis of Ensysce or the Proposed Business Combination. Viewers of this Presentation should each make their own evaluation of Ensysce and of the relevance and adequacy of the information and should make sure other investigations as they deem necessary. The information contained herein is as of February 3, 2020 and does not reflect any subsequent events. DISCLAIMER

Forward Looking Statements Certain statements included in this Presentation that are not historical facts are forward - looking statements for purposes of th e safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward - looking statements are sometimes accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “i ntend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward - loo king statements include, but are not limited to, statements regarding Ensysce’s business strategy, prospective milestones, cash resources and ability to obtain additional funding, current and prospective drug product candidates, planned cl inical trials and preclinical activities and potential product approvals, as well as the potential for market acceptance of any approved products and the related market opportunity. These statements are based on various assumptions, wh eth er or not identified in this press release, and on the current expectations of the respective management teams of Ensysce and Leisure and are not predictions of actual performance. These forward - looking statements are provided for il lustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are d iff icult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of Ensysce and Leisure. These forward - looking statements are subject to a number of risks and uncertainties, includi ng the risk that the potential product candidates that Ensysce develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; the risk that clinical trials may n ot confirm any safety, potency or other product characteristics described or assumed in this press release; the risk that Ensysce will be unable to successfully market or gain market acceptance of its product candidates; the risk that Ensysce ’s product candidates may not be beneficial to patients or successfully commercialized; the risk that Ensysce has overestimated the size of the target market, their willingness to try new therapies and the willingness of physicians to pres cri be these therapies; the effects of competition on Ensysce’s business; the risk that third parties on which Ensysce depends for laboratory, clinical development, manufacturing and other critical services will fail to perform satisfactorily; the risk that Ensysce’s business, operations, clinical development plans and timelines, and supply chain could be adversely affected by the effects of health epidemics, including the ongoing COVID - 19 pandemic; the risk that Ensysce will be un able to obtain and maintain sufficient intellectual property protection for its investigational products or will infringe the intellectual property protection of others; the potential inability of the parties to successfully or timely con sum mate the proposed business combination, including the risk that any regulatory approvals are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the combined company or the expected benefits of the proposed business combination or that the approval of the stockholders of Leisure is not obtained; the risk that Leisure is unable to maintain the listing of its securities on the Nasdaq stock market; the risk that proceeds from the $60 million forward equity purchase facility may be less than anticipated; the risk of failure to realize the anticipated benefits of the proposed business combination; the amount of redemption requests made by Leisure’s stockholders, and those factors discussed in Leisure’s Form 10 - K for the year ended December 31, 2019, under the heading “Risk Factors,” and other documents Leisure has filed, or will file, with the SEC, including a registration statement on Form S - 4 that will include a proxy statement/prospectus. If any of these risks materialize or Leisure’s and Ensysce’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward - looking statements . There may be additional risks that neither Leisure nor Ensysce presently know, or that neither Leisure nor Ensysce currently believe are immaterial, that could also cause actual results to differ from those contained in the forward - loo king statements. In addition, forward - looking statements do not reflect Leisure’s or Ensysce’s expectations, plans or forecasts of future events and views as of the date of this press release. Neither Leisure nor Ensysce anticipate that sub seq uent events and developments will cause Leisure’s and Ensysce’s assessments to change. However, while Leisure and Ensysce may elect to update these forward - looking statements at some point in the future, Leisure and Ensysce specifically d isclaim any obligation to do so. These forward - looking statements should not be relied upon as representing Leisure’s or Ensysce’s assessments of any date subsequent to the date of this press release. Accordingly, undue reliance shou ld not be placed upon the forward - looking statements. DISCLAIMER

Additional Information and Where to Find It In connection with the transaction described herein, Leisure intends to file relevant materials with the SEC, including a reg ist ration statement on Form S - 4, which will include a proxy statement/ prospectus. Promptly after the registration statement is declared effective by the SEC, Leisure will mail the definitive proxy statement/prospectus and a proxy card to e ach stockholder entitled to vote at the special meeting relating to the transaction. Investors and security holders of Leisure are urged to read these materials (including any amendments or supplements thereto) and any other relevant documents in connection with the transaction that Leisure will file with the SEC when they become available because they will contain important information about Leisure, Ensysce and the transaction. The preliminary proxy statement/prospectu s, the definitive proxy statement/prospectus and other relevant materials in connection with the transaction (when they become available), and any other documents filed by Leisure with the SEC, may be obtained free of charge at the SE C’s website (www.sec.gov). The documents filed by Leisure with the SEC also may be obtained free of charge at Leisure’s website at www.leisureacq.com or upon written request to Leisure at 250 West 57 th Street, Suite 415, New York, New York 10107, or by calling Leisure at (212) 565 - 6940. Participants in the Solicitation Leisure, Ensysce and their respective directors and executive officers may be deemed to be participants in the solicitation o f p roxies from Leisure’s shareholders in connection with the proposed transaction. Information about Leisure’s directors and executive officers and their ownership of Leisure’s securities is set forth in Leisure’s Definitive Proxy filed wi th the SEC on November 3, 2020. Additional information regarding the interests of those persons and other persons who may be deemed participants in the proposed transaction may be obtained by reading the proxy statement/prospectus regardin g t he proposed transaction when it becomes available. You may obtain free copies of these documents as described in the preceding paragraph. Non - Solicitation This Presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securitie s o r in respect of the potential transaction and shall not constitute an offer to sell or a solicitation of an offer to buy the securities of Leisure, the combined company or Ensysce, nor shall there be any sale of any such securities in any state o r j urisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeti ng the requirements of the Securities Act. DISCLAIMER
https://media - exp1.licdn.com/dms/image/C560BAQFJDJxIA426VQ/company - logo_200_200/0?e=1605744000&v=beta&t=KMNNYcZb2xGhs6jY9 - k1q5970xG4ChaU5Bfxk6kTtGU Presenters D. Lynn Kirkpatrick, PhD CEO of Ensysce • Co - founded 2 start up companies • Developed three targeted small molecule oncology drugs from discovery to clinic • Experience in private and public company raising funds from private, public and government sources 5 Daniel Silvers CEO of Leisure Lorne Weil Executive Chairman of Leisure • Executive leader and/or director of multiple SPAC successor entities • Led prior SPACs through successful acquisitions and integration • Accomplished Executive and Director with ability to navigate complex and uncertain environments • Renowned leader in the gaming sector with extensive experience in leading prior SPACs through successful acquisitions and integrations • Considerable transaction and operational experienced across a broad range of industries
6 I. Transaction Overview

Transaction Summary • Leisure Acquisition Corp. (“ LACQ ”) and Ensysce Biosciences, Inc. (“ Ensysce ”) have entered into a definitive merger agreement • Existing shareholders and convertible note holders of Ensysce will be rolling their entire interest into the combined Company • Ensysce existing shareholders expected to own approximately 71% of the outstanding common stock of the combined company at closing (1) • Transaction expected to be completed in Q2 2021 7 • Transaction values Ensysce Biosciences at an enterprise value of $207 million and is not subject to financing contingencies • Ensysce’s existing options and warrants would remain outstanding on their existing terms • Expected post transaction enterprise value of approximately $268 million based on a price of $10.00 per share . Transaction Summary Key Economic Terms Required Approvals Management and Independent Board • Lynn Kirkpatrick will continue role as CEO of the combined company • Leisure expected to appoint two directors and Ensysce expected to appoint five directors • LACQ and Ensysce shareholder approval • Registration statement effectiveness and approval for listing on NASDAQ Note: 1) Includes consideration to Ensysce common stock shareholders and convertible note holders (on an as - converted basis). Assumes no redemptions from LACQ’s existing public shareholders (as of 1/31/21).
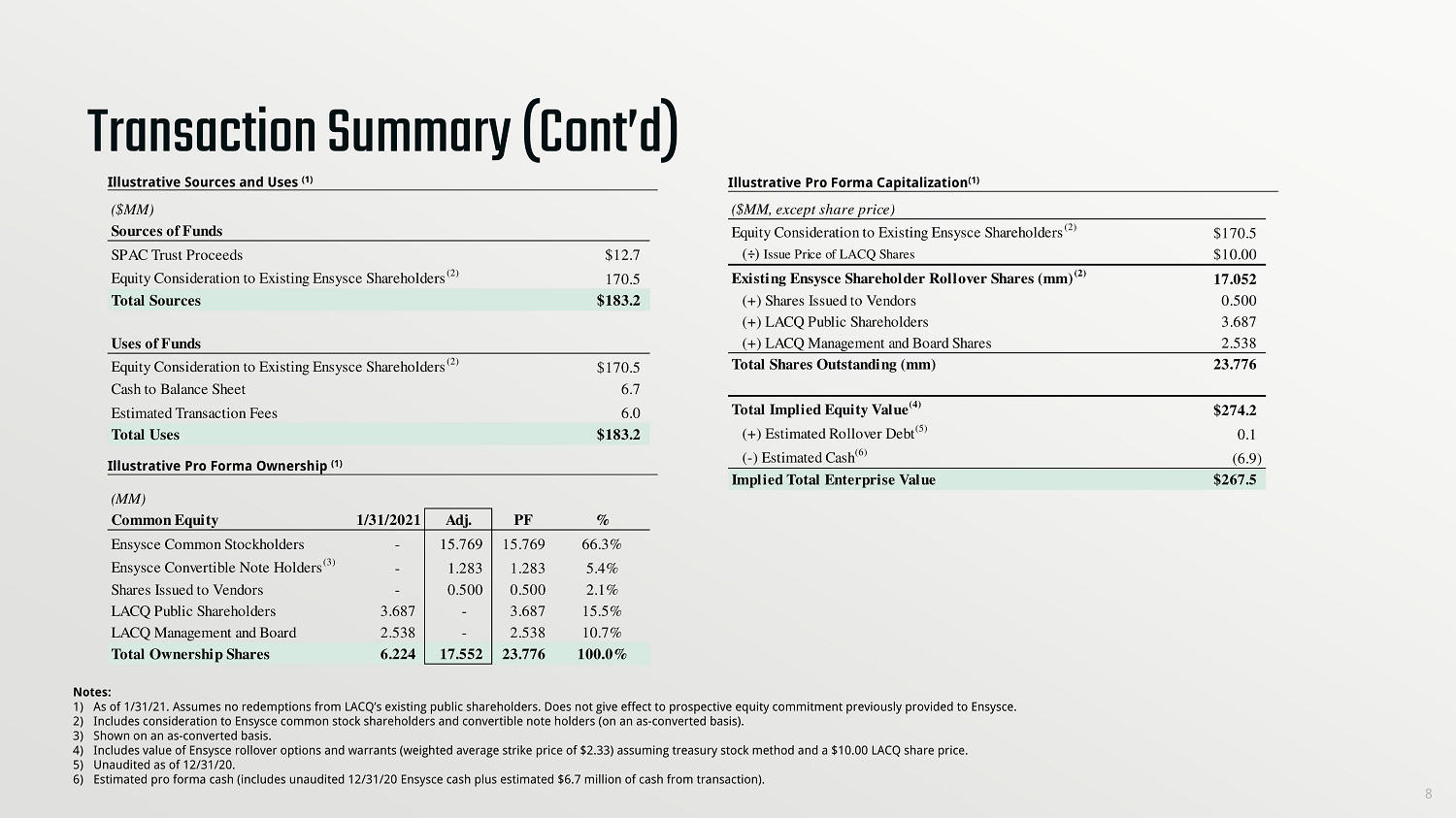
Transaction Summary (Cont’d) 8 Illustrative Sources and Uses (1) Illustrative Pro Forma Capitalization (1) Notes: 1) As of 1/31/21. Assumes no redemptions from LACQ’s existing public shareholders. Does not give effect to prospective equity co mmi tment previously provided to Ensysce. 2) Includes consideration to Ensysce common stock shareholders and convertible note holders (on an as - converted basis). 3) Shown on an as - converted basis. 4) Includes value of Ensysce rollover options and warrants (weighted average strike price of $2.33) assuming treasury stock meth od and a $10.00 LACQ share price. 5) Unaudited as of 12/31/20. 6) Estimated pro forma cash (includes unaudited 12/31/20 Ensysce cash plus estimated $6.7 million of cash from transaction). Illustrative Pro Forma Ownership (1) ($MM) Sources of Funds SPAC Trust Proceeds $12.7 Equity Consideration to Existing Ensysce Shareholders (2) 170.5 Total Sources $183.2 Uses of Funds Equity Consideration to Existing Ensysce Shareholders (2) $170.5 Cash to Balance Sheet 6.7 Estimated Transaction Fees 6.0 Total Uses $183.2 (MM) Common Equity 1/31/2021 Adj. PF % Ensysce Common Stockholders - 15.769 15.769 66.3% Ensysce Convertible Note Holders (3) - 1.283 1.283 5.4% Shares Issued to Vendors - 0.500 0.500 2.1% LACQ Public Shareholders 3.687 - 3.687 15.5% LACQ Management and Board 2.538 - 2.538 10.7% Total Ownership Shares 6.224 17.552 23.776 100.0%

Anticipated Transaction Timeline • Transaction Agreement Executed and Announced 9 January/February 2021 March 2021 Second Quarter 2021 • Set Record Date for Shareholder Vote • Expected Mailing of Final Proxy Materials to Shareholders • Hold Shareholder Vote and Anticipated Close of Transaction • Preliminary Proxy Materials Filed with the SEC

10 II. Ensysce Overview

ENSYSCE OVERVIEW Ensysce is a clinical - stage drug company using its proprietary prodrug technology platform to improve safety of prescription drugs. First focus is to develop an entirely new class of powerful, tamper - proof opioids that prevent both drug abuse and drug overdoses 11 • CLINICAL STAGE COMPANY: • Two new , revolutionary platforms that aim to eliminate opioid abuse (TAAP) and prevent drug overdose (MPAR) • Repurposed protease inhibitor program for a COVID - 19 Therapeutic and Cystic Fibrosis . • FDA FAST TRACK: lead drug product PF614 • NIH/NIDA govt awards: major funding through 2024 (1) • NEW CLASS OF PAIN DRUGS TO LAUNCH 2024 Note: 1) A portion of funding subject to reaching clinical development milestones.
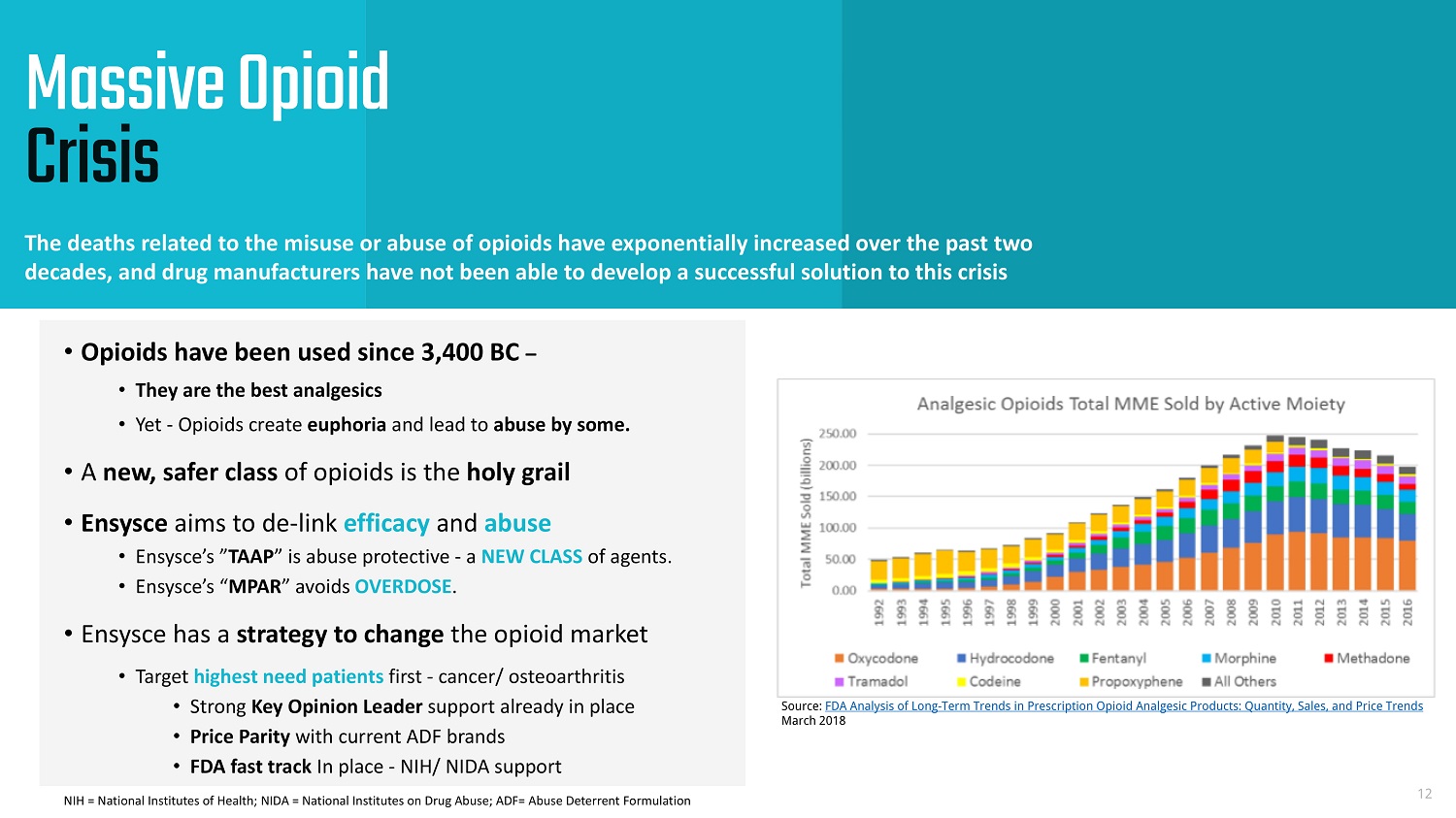
Massive Opioid Crisis The deaths related to the misuse or abuse of opioids have exponentially increased over the past two decades, and drug manufacturers have not been able to develop a successful solution to this crisis 12 Source: FDA Analysis of Long - Term Trends in Prescription Opioid Analgesic Products: Quantity, Sales, and Price Trends March 2018
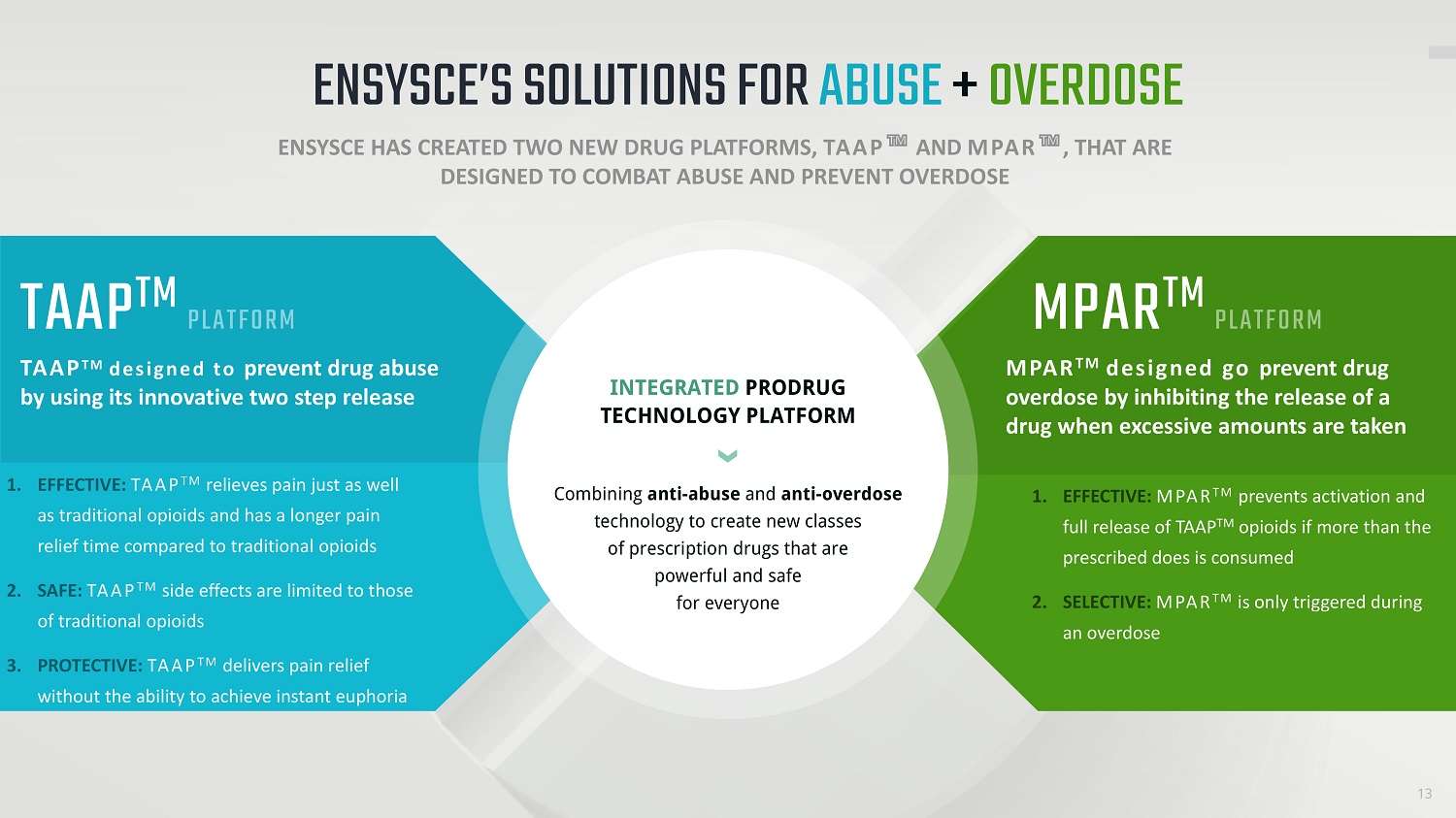
MPAR TM designed go prevent drug overdose by inhibiting the release of a drug when excessive amounts are taken ENSYSCE’S SOLUTIONS FOR ABUSE + OVERDOSE 13 MPAR TM Combining anti - abuse and anti - overdose technology to create new classes of prescription drugs that are powerful and safe for everyone INTEGRATED PRODRUG TECHNOLOGY PLATFORM 1. EFFECTIVE: TAAP TM relieves pain just as well as traditional opioids and has a longer pain relief time compared to traditional opioids 2. SAFE: TAAP TM side effects are limited to those of traditional opioids 3. PROTECTIVE: TAAP TM delivers pain relief without the ability to achieve instant euphoria 1. EFFECTIVE: MPAR TM prevents activation and full release of TAAP TM opioids if more than the prescribed does is consumed 2. SELECTIVE: MPAR TM is only triggered during an overdose TAAP TM designed to prevent drug abuse by using its innovative two step release TAAP TM ENSYSCE HAS CREATED TWO NEW DRUG PLATFORMS, TAAP ༩ AND MPAR ༩ , THAT ARE DESIGNED TO COMBAT ABUSE AND PREVENT OVERDOSE

LOW CNS PENETRATION thereby limiting the amount of drug in the brain to bind to receptors and preventing euphoric side effect Opioid Receptor Binding PF614 LOW AFFINITY: without GI activation, reducing the binding to receptors and hence, minimal side effect INACTIVE if inhaled or injected CANNOT BE MANIPULATED to provide active opioid immediately; must be swallowed and released in gastrointestinal tract, thereby preventing abuse 12 HOURS , truly twice a day CNS Penetration Inhalation / Injection Manipulation Half - Life VS 14 TAAP TM – modified oxycodone provides longer pain relief and a lower risk for abuse than the traditional oxycodone ENSYSCE SOLUTION VS. THE COMPETITION Oxycodone HIGH AFFINITY: increasing the strength of binding to receptors and hence, promoting pleasure (euphoria) HIGH CNS PENETRATION thereby increasing the amount of drug in the brain to bind to receptors and providing euphoria ACTIVE IMMEDIATELY if inhaled or injected CAN BE MANIPULATED by crushing to provide active opioid immediately and therefore, opioid abuse 5 - 7 HOURS , yet prescribed twice a day
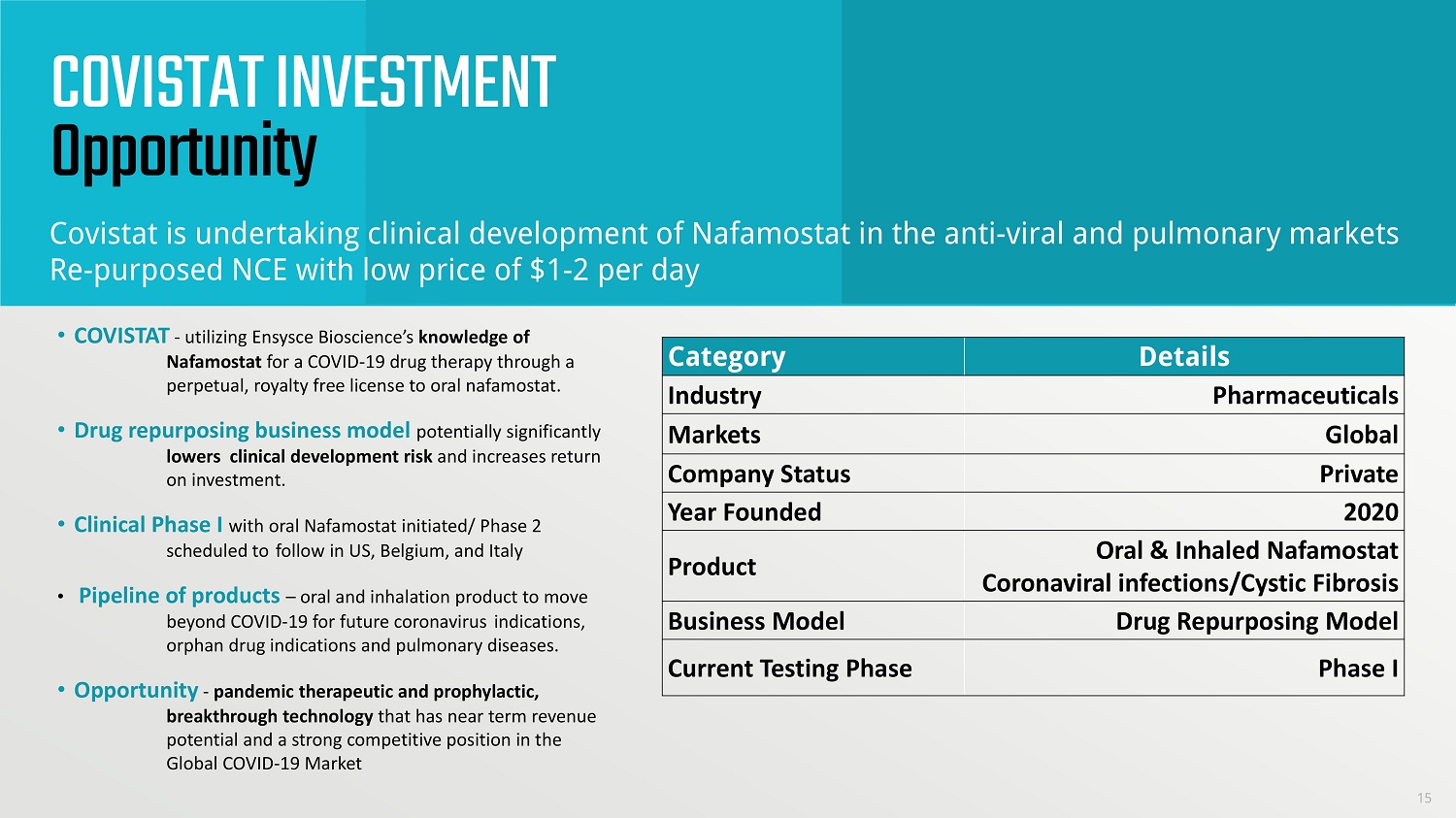
COVISTAT INVESTMENT Opportunity • COVISTAT - utilizing Ensysce Bioscience’s knowledge of Nafamostat for a COVID - 19 drug therapy through a perpetual, royalty free license to oral nafamostat. • Drug repurposing business model potentially significantly lowers clinical development risk and increases return on investment. • Clinical Phase I with oral Nafamostat initiated/ Phase 2 scheduled to follow in US, Belgium, and Italy • Pipeline of products – oral and inhalation product to move beyond COVID - 19 for future coronavirus indications, orphan drug indications and pulmonary diseases. • Opportunity - pandemic therapeutic and prophylactic, breakthrough technology that has near term revenue potential and a strong competitive position in the Global COVID - 19 Market Category Details Industry Pharmaceuticals Markets Global Company Status Private Year Founded 2020 Product Oral & Inhaled Nafamostat Coronaviral infections/Cystic Fibrosis Business Model Drug Repurposing Model Current Testing Phase Phase I Covistat is undertaking clinical development of Nafamostat in the anti - viral and pulmonary markets Re - purposed NCE with low price of $1 - 2 per day

16 III. Key Investment Highlights

Key Investment Highlights Revolutionary Abuse - Resistant Opioids – Ensysce has developed a breakthrough technology to produce opioids in a manner that can provide effective pain - relief without allowing for abuse. C 17 Successful Phase I Data – Phase I data have demonstrated Ensysce’s opioid PF614 as abuse - resistant and safe without compromising on efficacy; PF614 expected to launch by 2024 generating revenue for ongoing programs. De - Risked and Accelerated FDA Milestones – Ensysce has secured FDA Fast - Track Designation and is using the 505(b)(2) regulatory pathway, substantially reducing the trial/regulatory risk and potential time to market. NIH/NIDA Grant of $23MM – Ensysce has been awarded a coveted $23MM non - dilutive grant in funding from National Institute on Drug Abuse (NIDA) and National Institute of Health (NIH) , helping accelerate the Company’s development pipeline and reducing equity required from investors. Breakthrough Technology Well - Protected by Patents – Ensysce has over 100 patents already issued in 25 countries, ensuring a barrier to entry from new competitors globally. Huge Unmet Need – Currently, there are virtually no opioids that can be prescribed without abuse and overdose potential. There are no low - cost therapeutic agents to treat COVID - 19. Well - Rounded Seasoned Management – Ensysce has an experienced leadership team with significant expertise and experience in all facets of biotech company - building, from drug development to commercialization . C C

HUGE UNMET NEED The chronic pain market with opioid indication has a huge unmet need with virtually no effective opioids that can be prescribed without abuse and overdose potential 18 Massive Market • Annual U.S. opioid is an attractive market of approximately $18.4 (1) Billion with opioid prescriptions constituting more than half of the total prescription pain market (2 ) 153 million opioid prescriptions every year. (3 ) Abuse - Deterrent Formulas are Ineffective • The current abuse - deterrent opioids have not been delivering true deterrence and combatting abuse since they are mere physical modulations and can be easily crushed and abused, signaling the huge unmet need for better alternatives. Opioid Restriction is Damaging • Denying people who rely on opioids to successfully manage chronic pain may cause them to regress into pain and unable to work . Frequently desperate to alleviate pain caused by serious medical conditions, chronic pain sufferers may turn to street drugs or even suicide. MORE PAIN MORE PAIN KILLERS MORE PAIN KILLERS THAT KILL
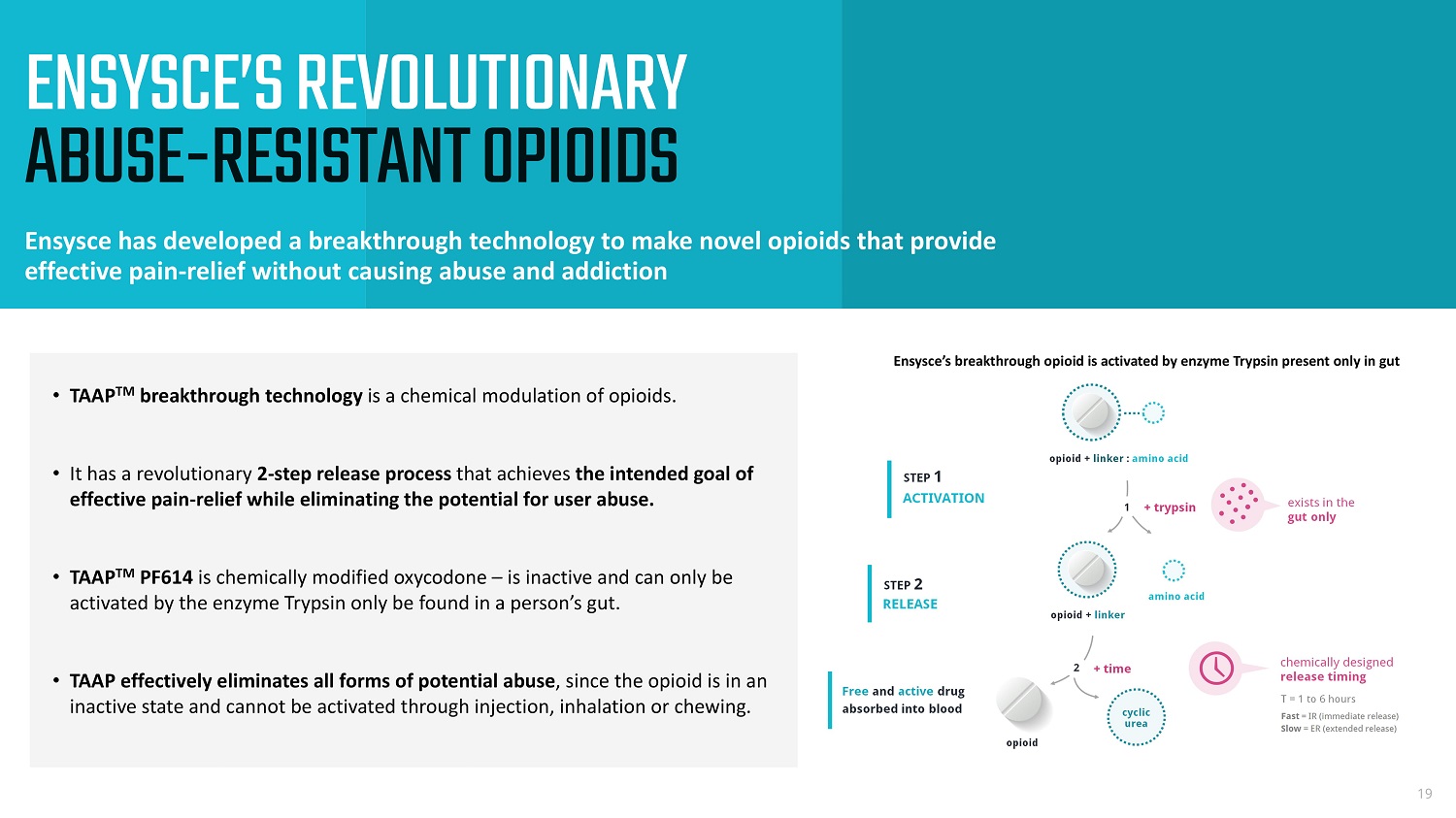
ENSYSCE’S REVOLUTIONARY ABUSE - RESISTANT OPIOIDS Ensysce has developed a breakthrough technology to make novel opioids that provide effective pain - relief without causing abuse and addiction 19 Ensysce’s breakthrough opioid is activated by enzyme Trypsin present only in gut • TAAP TM breakthrough technology is a chemical modulation of opioids. • It has a revolutionary 2 - step release process that achieves the intended goal of effective pain - relief while eliminating the potential for user abuse. • TAAP TM PF614 is chemically modified oxycodone – is inactive and can only be activated by the enzyme Trypsin only be found in a person’s gut. • TAAP effectively eliminates all forms of potential abuse , since the opioid is in an inactive state and cannot be activated through injection, inhalation or chewing.
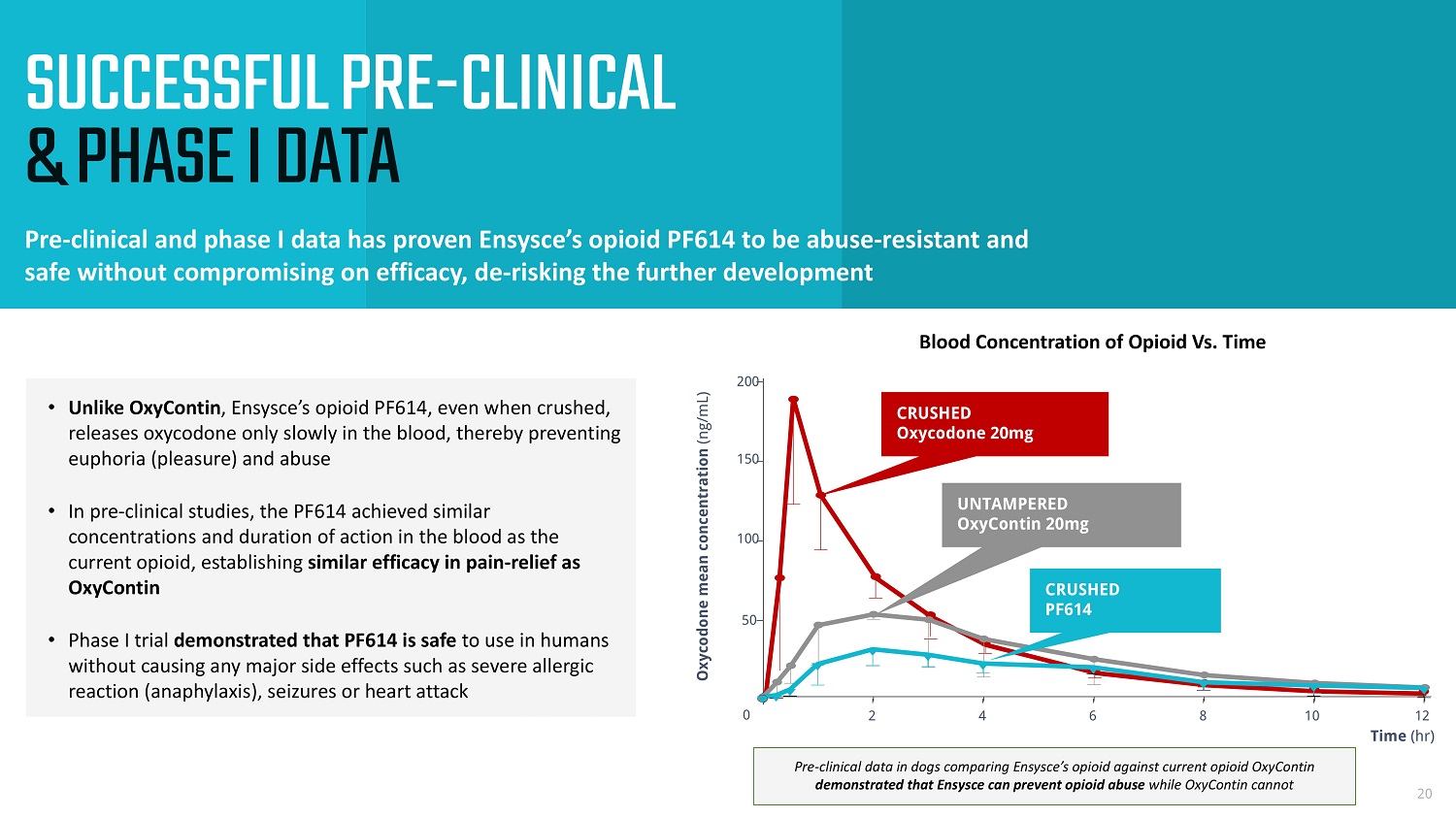
SUCCESSFUL PRE - CLINICAL & PHASE I DATA Pre - clinical and phase I data has proven Ensysce’s opioid PF614 to be abuse - resistant and safe without compromising on efficacy, de - risking the further development 20 Time (hr) Oxycodone mean concentration (ng/mL) 0 2 4 6 8 10 12 0 50 100 150 200 UNTAMPERED OxyContin 20mg CRUSHED PF 614 CRUSHED Oxycodone 20mg • Unlike OxyContin , Ensysce’s opioid PF614, even when crushed, releases oxycodone only slowly in the blood, thereby preventing euphoria (pleasure) and abuse • In pre - clinical studies, the PF614 achieved similar concentrations and duration of action in the blood as the current opioid, establishing similar efficacy in pain - relief as OxyContin • Phase I trial demonstrated that PF614 is safe to use in humans without causing any major side effects such as severe allergic reaction (anaphylaxis), seizures or heart attack Blood Concentration of Opioid Vs. Time Pre - clinical data in dogs comparing Ensysce’s opioid against current opioid OxyContin demonstrated that Ensysce can prevent opioid abuse while OxyContin cannot

DE - RISKED AND ACCELERATED FDA MILESTONES Ensysce has secured FDA Fast - Track Designation and is using the 505(b)(2) regulatory pathway, substantially reducing the trial/regulatory risk and time to market 21 • 505(b)(2) regulatory pathway status from the FDA and successful Phase I data de - risk the regulatory path , since Ensysce can submit bioequivalence data demonstrating the efficacy of the already - proven reference drug oxycodone on the market, drastically increasing the probability of success and reducing costs for Phase 3 trials • Ensysce’s PF 614 has secured Fast Track designation from the FDA, enabling ongoing communication and collaboration with the FDA in all stages of drug - development with eligibility for accelerated approval , significantly reducing the costs and time to market in 2023 • Ensysce has completed a preliminary study with the leading abuse - testing lab in the U.S. This study showed that PF614 could not be “cracked” by kitchen chemistry, a unique finding which no ADF has been able to achieve • Additionally, Human Abuse Liability (HAL) and Kitchen Chemistry Test designed with FDA’s guidance would enable Ensysce to get Abuse Deterrent Labelling and differentiate Ensysce’s opioid PF614 from OxyContin

COVETED, NON - DILUTIVE NIH/NIDA GRANT OF $23MM Ensysce has been awarded coveted $23MM non - dilutive funding by NIH/NIDA, providing recognition to Ensysce’s technologies in combating abuse and reducing equity risk for investors (1) 22 • The NIH/NIDA funding is a testament of credibility and recognition by NIDA, NIH and the federal government of the value of Ensysce’s technologies (TAAP TM and MPAR TM ) in preventing opioid abuse and overdose. • In 2018, Ensysce received $9MM of NIDA grant for pre - clinical and Phase I development of MPAR TM technology to prevent opioid overdose • In Dec. 2019, Ensysce was awarded an additional $14.5MM under the NIH’s HEAL Initiative for pre - clinical and Phase I development of Opioid Use Disorder treatment (TAAP TM Methadone) using Ensysce’s TAAP TM and MPAR TM overdose protection platforms. • NIH/NIDA grants provide non - dilutive sources of capital for development of the assets in the pipeline, potentially reducing equity risk and increasing the upside on invested capital for equity investors. Note: 1) A portion of funding is predicated on Ensysce achieving agreed upon clinical development milestones.

Ensysce has over 100 patents already issued in 25 countries, ensuring barriers to entry for new companies globally 23 • Ensysce’s technology is well - protected by a suite of 111 patents i ssued in the U.S. and overseas (the UK, a majority of the EU, Australia, China, and others with a total of 25 countries), ensuring a barrier to entry for other companies in these markets. • These patents provide protection to the underlying molecules of both the immediate and extended release formulations of Ensysce. • Ensysce patent pipeline will grow with a number of new products in development, has a library of trade secrets and trademarks. Color coded regions on the map indicate countries where patents have been issued EXTENSIVE PATENT PORTFOLIO

Ensysce’s leadership has significant experience in all facets of biotech company - building, from drug development to commercialization 24 • Ensysce’s team is led by CEO Lynn Kirkpatrick, who is a serial biotech entrepreneur with over 100 publications in peer - reviewed journals and a featured biotech superstar. • Ensysce has assembled an extraordinary team with an extensive track record in leading teams and taking drugs through development, regulatory approvals, capital raise and commercialization in both public and private companies. • Ensysce has an outstanding advisory team which includes some of the leading opioid experts in the world with decades of experience in pain medication and opioid abuse. Previous Experience WELL - ROUNDED MANAGEMENT TEAM
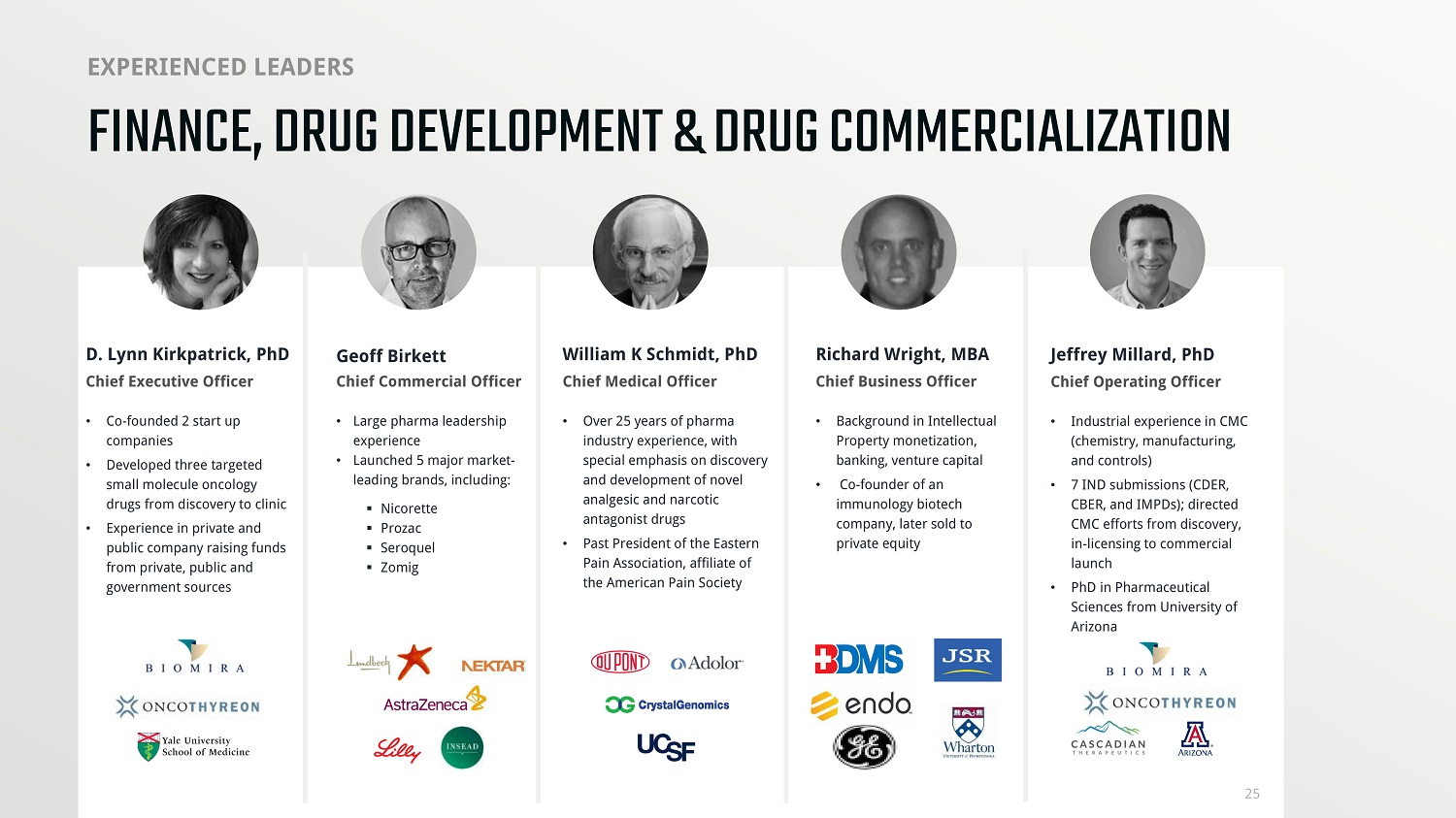
https://media - exp1.licdn.com/dms/image/C560BAQFJDJxIA426VQ/company - logo_200_200/0?e=1605744000&v=beta&t=KMNNYcZb2xGhs6jY9 - k1q5970xG4ChaU5Bfxk6kTtGU FINANCE, DRUG DEVELOPMENT & DRUG COMMERCIALIZATION EXPERIENCED LEADERS D. Lynn Kirkpatrick, PhD Chief Executive Officer Chief Medical Officer William K Schmidt, PhD Chief Commercial Officer Geoff Birkett Richard Wright, MBA Chief Business Officer • Large pharma leadership experience • Launched 5 major market - leading brands, including: ▪ Nicorette ▪ Prozac ▪ Seroquel ▪ Zomig • Co - founded 2 start up companies • Developed three targeted small molecule oncology drugs from discovery to clinic • Experience in private and public company raising funds from private, public and government sources • Over 25 years of pharma industry experience, with special emphasis on discovery and development of novel analgesic and narcotic antagonist drugs • Past President of the Eastern Pain Association, affiliate of the American Pain Society • Background in Intellectual Property monetization, banking, venture capital • Co - founder of an immunology biotech company, later sold to private equity 25 Jeffrey Millard, PhD Chief Operating Officer • Industrial experience in CMC (chemistry, manufacturing, and controls) • 7 IND submissions (CDER, CBER, and IMPDs); directed CMC efforts from discovery, in - licensing to commercial launch • PhD in Pharmaceutical Sciences from University of Arizona

26 IV. Ensysce Forward Looking Projections

2020 2021 2022 2023 PIPELINE 27 CLINICAL IND NDA • CMC preparation for HAL and bioequivalence. • MPAR TM and OUD preclinical program initated. • Nafamostat Phase 1 trial. • MPAR TM IND submission. • PF614 Human Abuse Liability (HAL) clinical trial: nasal and oral. • PF614 bioequivalence (Phase 2) data readout (505(b)(2) approval). • PF614 - MPAR Phase 1 trial. • Nafamostat – Phase 2 in COVID 19 • PF614 Phase 3 Trial: chronic pain. • Develop MPAR TM overdose protection Phase 1 pipeline. • OUD methadone IND. • PF614 NDA submission • Progress pipeline technology clinical development • Expand TAAP TM platform licensing partnerships PIPELINE CLINICAL PIPELINE CLINICAL PIPELINE CLINICAL IND Ensysce is on target to deliver commercial launch of its PF614 drug by 2024 Key Milestones CMC = Chemistry manufacturing and controls MPAR = overdose protection IND = Investigational New drug application to FDA OUD = opioid use disorder HAL = human abuse liability studies Designates product in development Designates IND submitted
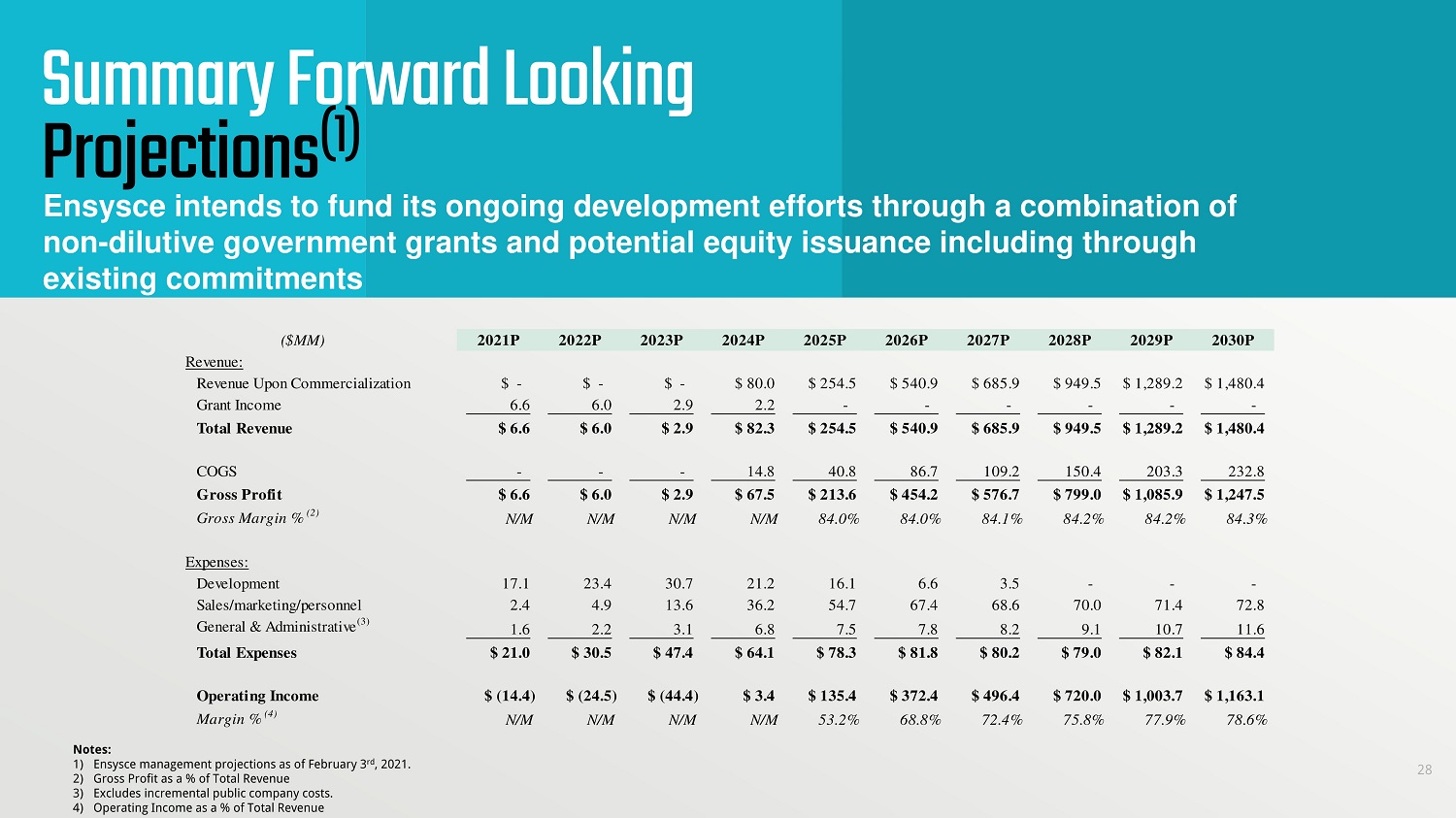
28 Ensysce intends to fund its ongoing development efforts through a combination of non - dilutive government grants and potential equity issuance including through existing commitments Summary Forward Looking Projections (1) ($MM) 2021P 2022P 2023P 2024P 2025P 2026P 2027P 2028P 2029P 2030P Revenue: Revenue Upon Commercialization $ - $ - $ - $ 80.0 $ 254.5 $ 540.9 $ 685.9 $ 949.5 $ 1,289.2 $ 1,480.4 Grant Income 6.6 6.0 2.9 2.2 - - - - - - Total Revenue $ 6.6 $ 6.0 $ 2.9 $ 82.3 $ 254.5 $ 540.9 $ 685.9 $ 949.5 $ 1,289.2 $ 1,480.4 COGS - - - 14.8 40.8 86.7 109.2 150.4 203.3 232.8 Gross Profit $ 6.6 $ 6.0 $ 2.9 $ 67.5 $ 213.6 $ 454.2 $ 576.7 $ 799.0 $ 1,085.9 $ 1,247.5 Gross Margin % (2) N/M N/M N/M N/M 84.0% 84.0% 84.1% 84.2% 84.2% 84.3% Expenses: Development 17.1 23.4 30.7 21.2 16.1 6.6 3.5 - - - Sales/marketing/personnel 2.4 4.9 13.6 36.2 54.7 67.4 68.6 70.0 71.4 72.8 General & Administrative (3) 1.6 2.2 3.1 6.8 7.5 7.8 8.2 9.1 10.7 11.6 Total Expenses $ 21.0 $ 30.5 $ 47.4 $ 64.1 $ 78.3 $ 81.8 $ 80.2 $ 79.0 $ 82.1 $ 84.4 Operating Income $ (14.4) $ (24.5) $ (44.4) $ 3.4 $ 135.4 $ 372.4 $ 496.4 $ 720.0 $ 1,003.7 $ 1,163.1 Margin % (4) N/M N/M N/M N/M 53.2% 68.8% 72.4% 75.8% 77.9% 78.6%
29 V. Appendices
30 Ensysce Product Pipeline
TAMPER - PROOF ANTI - ABUSE TM TAAP TM is only activated by trypsin , a digestive enzyme that exists only in the gut; therefore crushing, inhaling or injecting it will not cause the opioid to be released faster to produce pleasure/euphoria 31 TAAP TM chemically modifies the opioid, thereby eliminating the potential abuse by the patient through physical means (e.g., crushing and subsequent injection)

The picture can't be displayed. 32 TWO - STEP RELEASE PROCESS opioid + linker : amino acid opioid + linker + trypsin amino acid opioid cyclic urea chemically designed release timing T = 1 to 6 hours Fast = IR (immediate release) Slow = ER (extended release) Free and active drug absorbed into blood 1 2 ACTIVATION STEP 1 RELEASE STEP 2 exists in the gut only + time TAAP TM MECHANISM OF ACTION • The drug is activated by the enzyme trypsin only when it reaches the gut, preventing abuse outside the body • The inactive drug releases active oxycodone gradually in the blood, preventing immediate onset and abuse

SMART ANTI - OVERDOSE The prescribed activity of trypsin will not be affected by MPAR TM , therefore making it very safe for daily use TM MPAR TM inhibits trypsin when too much TAAP TM opioid is swallowed, inhibiting full activation and opioid release, and therefore, preventing overdose - related deaths 33 MPAR TM is only triggered by an overdose , blocking the additional doses consumed
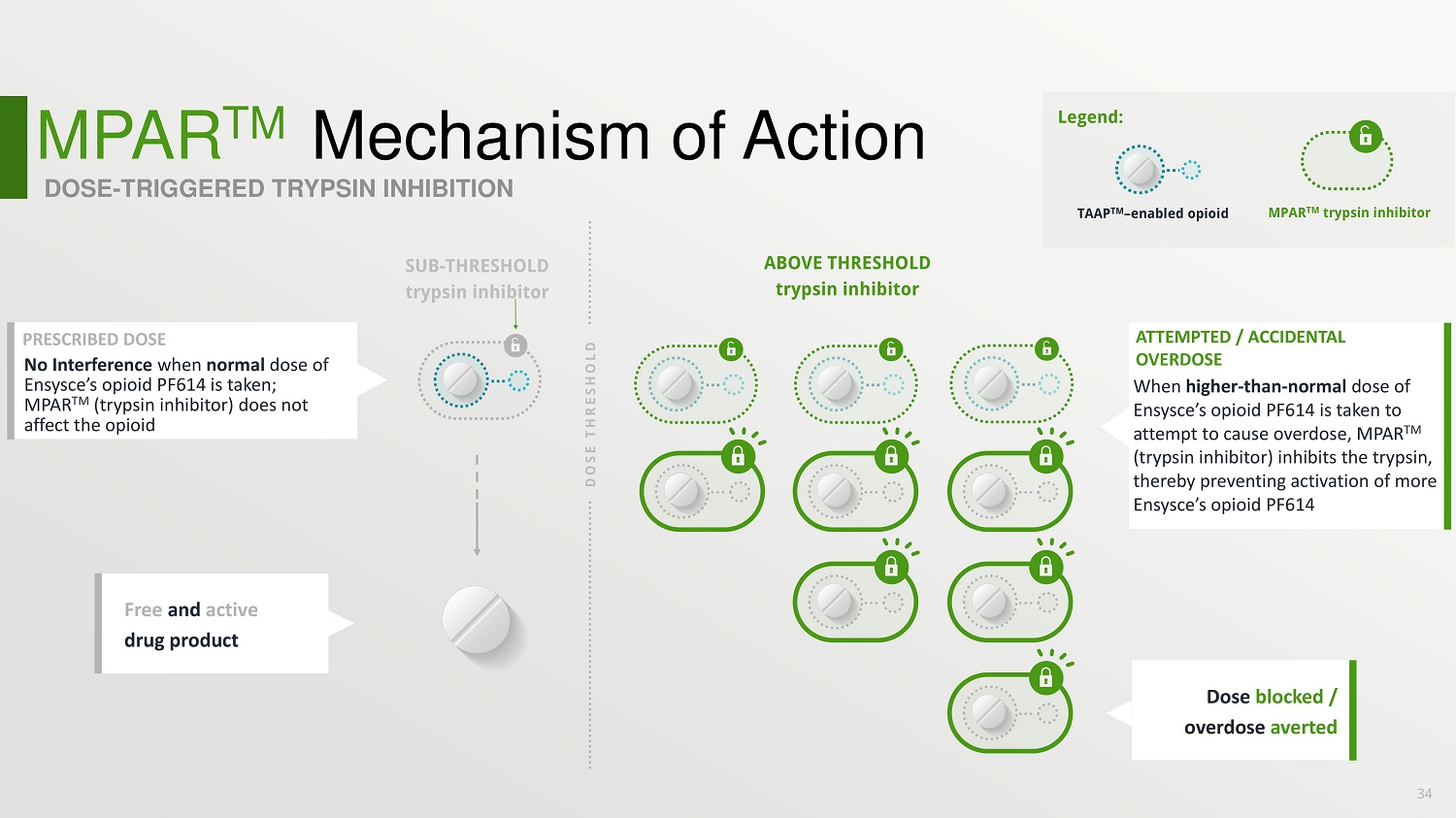
MPAR TM Mechanism of Action DOSE - TRIGGERED TRYPSIN INHIBITION ABOVE THRESHOLD trypsin inhibitor ATTEMPTED / ACCIDENTAL OVERDOSE Free and active drug product Dose blocked / overdose averted 34 PRESCRIBED DOSE No Interference when normal dose of Ensysce’s opioid PF614 is taken; MPAR TM (trypsin inhibitor) does not affect the opioid TAAP TM – enabled opioid MPAR TM trypsin inhibitor Legend: When higher - than - normal dose of Ensysce’s opioid PF614 is taken to attempt to cause overdose, MPAR TM (trypsin inhibitor) inhibits the trypsin, thereby preventing activation of more Ensysce’s opioid PF614
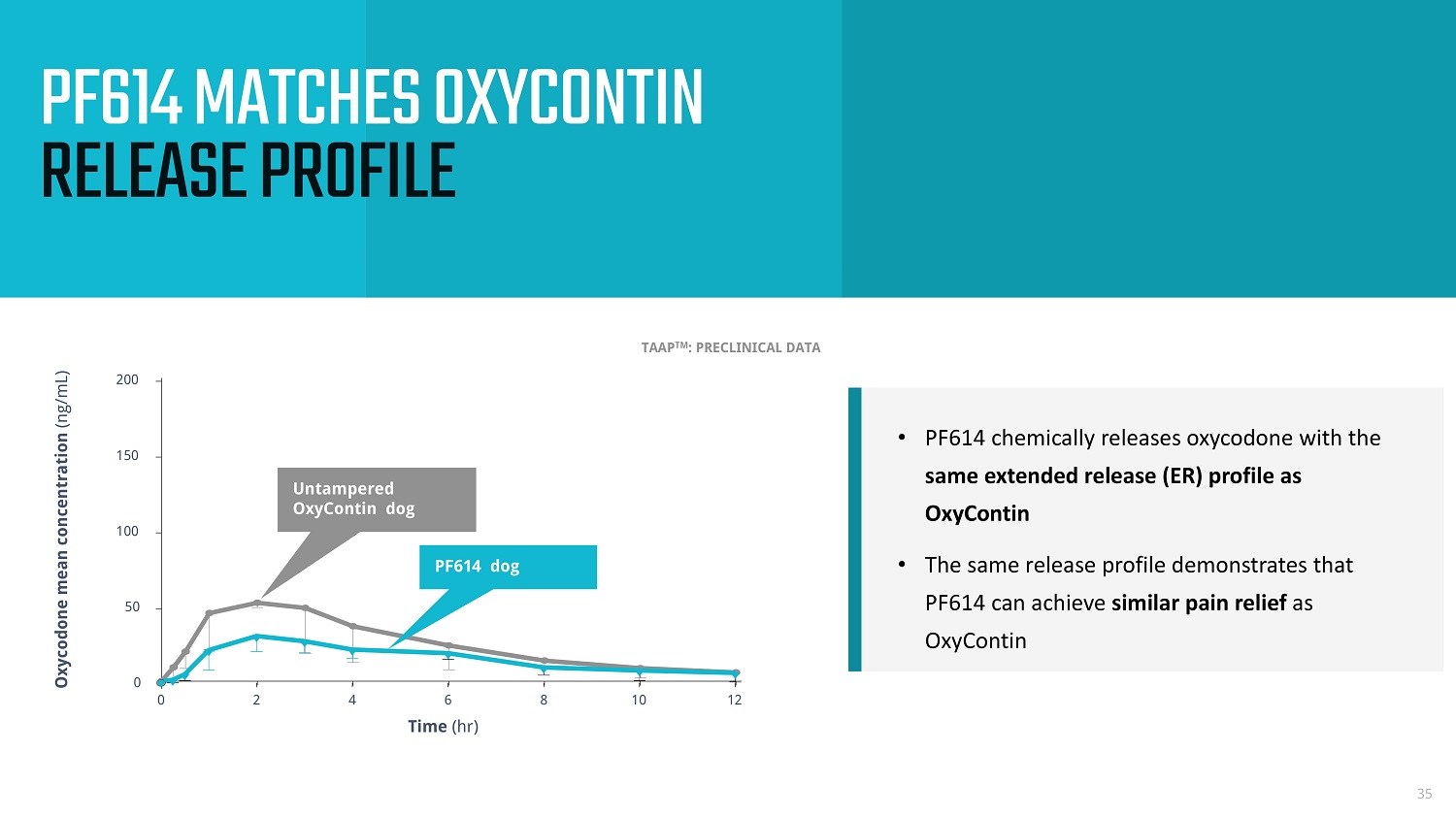
0 2 4 6 8 10 12 0 50 100 150 200 Time (hr) Oxycodone mean concentration (ng/mL) • PF614 chemically releases oxycodone with the same extended release (ER) profile as OxyContin • The same release profile demonstrates that PF614 can achieve similar pain relief as OxyContin 35 Untampered OxyContin dog PF614 dog PF614 MATCHES OXYCONTIN RELEASE PROFILE TAAP TM : PRECLINICAL DATA

ABUSE PREVENTION • As show in the graph on the left, the onset of Ensysce’s PF614 in blood is slow even at higher doses, demonstrating the ability to prevent opioid pleasure (euphoria) and abuse SAFE • PF614 has shown to be safe , and no unexpected adverse events were observed in Phase I EFFICIENT CONVERSION TO OXYCODONE • PF614 is effectively converted to Oxycodone with an efficiency of 90% , thereby replicating the pain - relief by OxyContin (oxycodone) 36 PF614: CLINICALLY PROVEN SAFER, EFFIECENT & LONGER - LASTING PAIN RELIEF TAAP TM : CLINICAL DATA Oxycodone concentration in Blood vs. Time SLOW ONSET max blood levels reached @ 4 – 6h across all cohorts 15 mg / 21 / no / fasted 15 mg / 22 / yes / fasted 25 mg / 6 / yes / fasted 50 mg / 6 / yes / fasted 100 mg / 6 / yes / fasted 200 mg / 6 / yes / fasted 200 mg / 8 / yes / high - fat Dose / n / Naltrexone / meal 0 6 12 18 24 30 36 0 20 40 60 80 100 Time (hr) Oxycodone mean concentration (ng/mL)
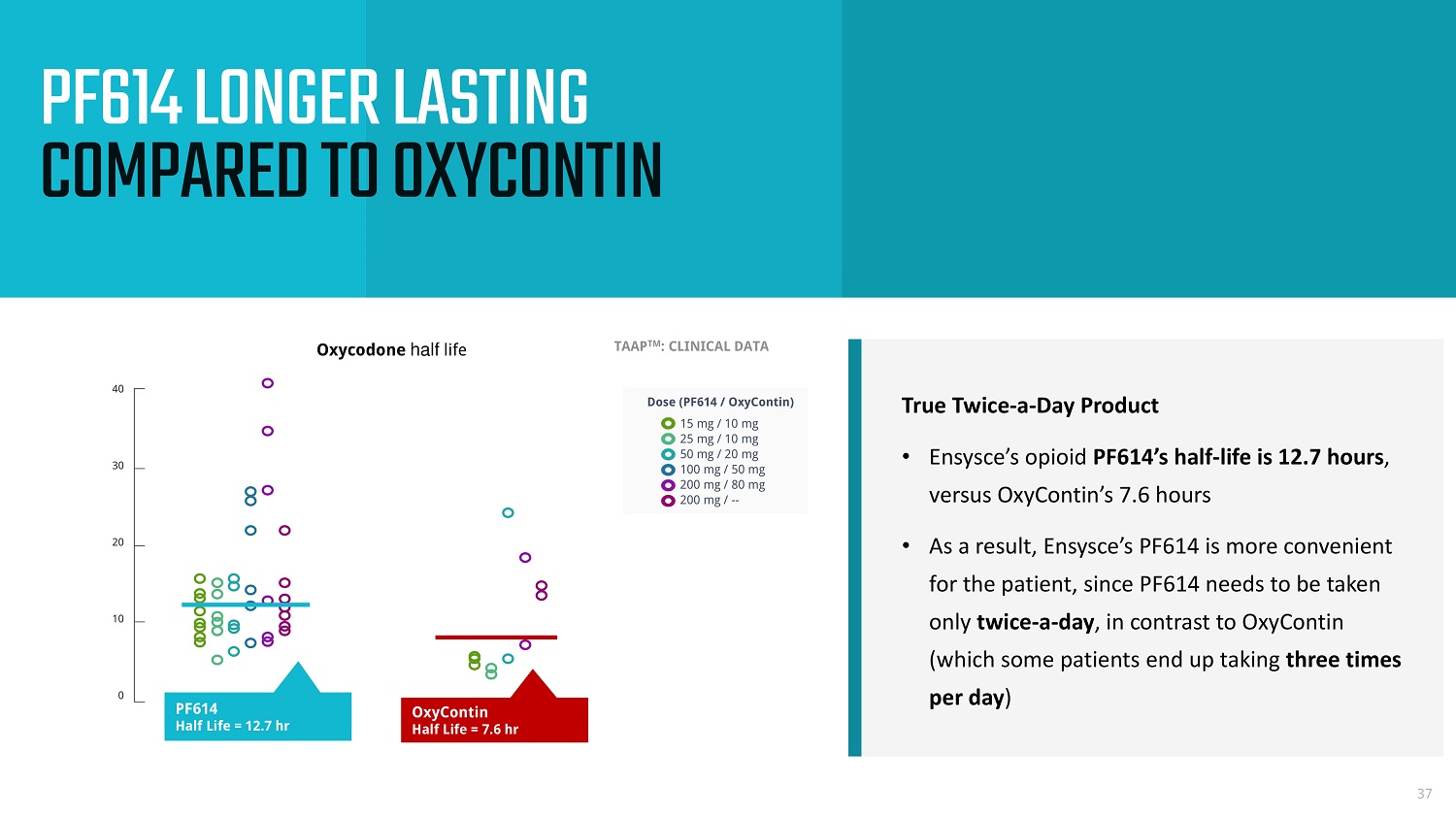
True Twice - a - Day Product • Ensysce’s opioid PF614’s half - life is 12.7 hours , versus OxyContin’s 7.6 hours • As a result, Ensysce’s PF614 is more convenient for the patient, since PF614 needs to be taken only twice - a - day , in contrast to OxyContin (which some patients end up taking three times per day ) 37 PF614 LONGER LASTING COMPARED TO OXYCONTIN TAAP TM : CLINICAL DATA Oxycodone half life The picture can't be display ed. 15 mg / 10 mg 25 mg / 10 mg 50 mg / 20 mg 100 mg / 50 mg 200 mg / 80 mg 200 mg / -- Dose (PF614 / OxyContin) 0 10 20 30 40 OxyContin Half Life = 7.6 hr PF614 Half Life = 12.7 hr
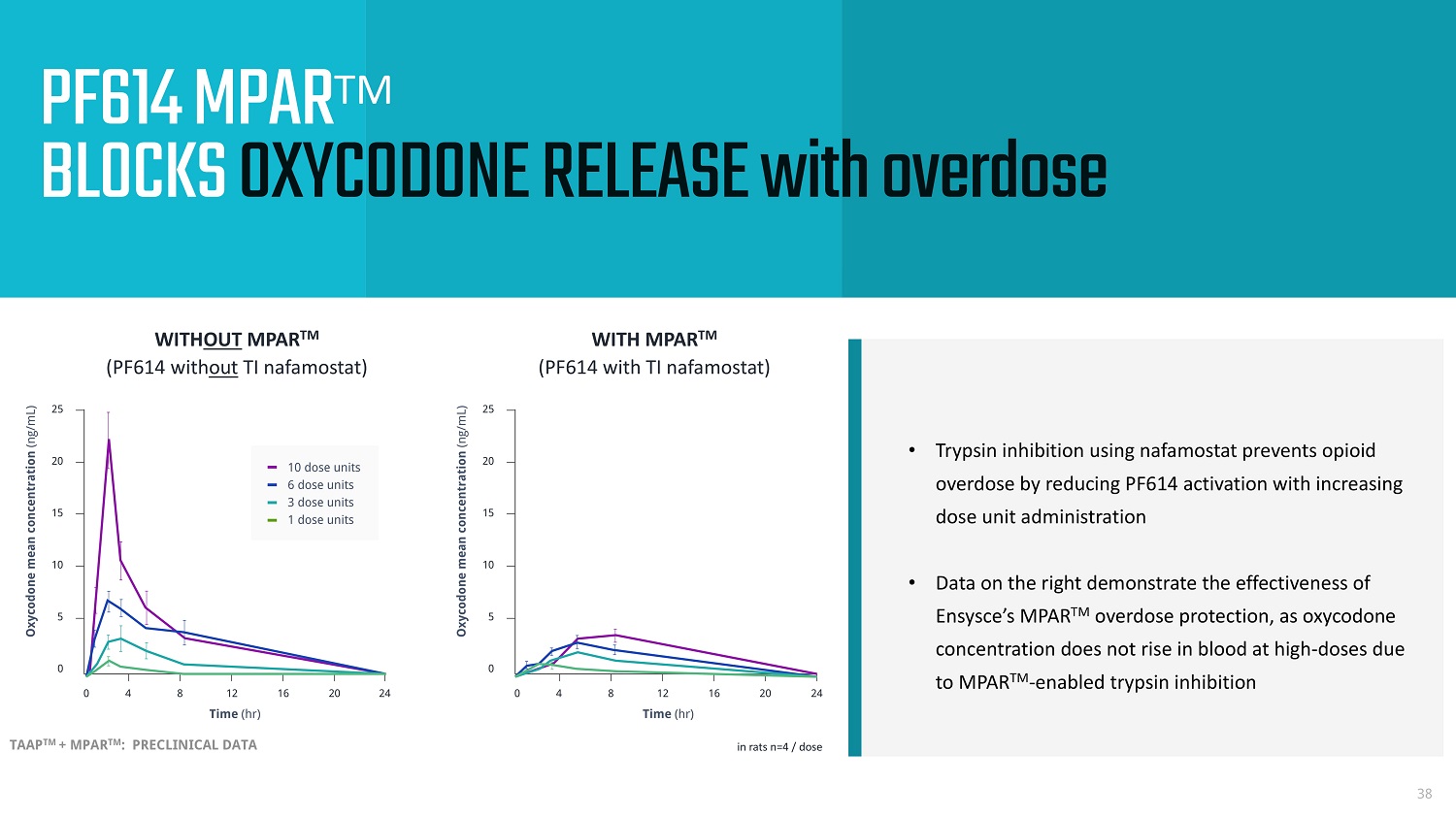
• Trypsin inhibition using nafamostat prevents opioid overdose by reducing PF614 activation with increasing dose unit administration • Data on the right demonstrate the effectiveness of Ensysce’s MPAR TM overdose protection, as oxycodone concentration does not rise in blood at high - doses due to MPAR TM - enabled trypsin inhibition 38 PF614 MPAR TM BLOCKS OXYCODONE RELEASE with overdose TAAP TM + MPAR TM : PRECLINICAL DATA WITH MPAR TM (PF614 with TI nafamostat) WITH OUT MPAR TM (PF614 with out TI nafamostat) Oxycodone mean concentration (ng/mL) Time (hr) 0 4 8 12 16 24 0 5 10 15 20 25 20 0 4 8 12 16 24 0 5 10 15 20 25 Time (hr) Oxycodone mean concentration (ng/mL) 20 10 dose units 6 dose units 3 dose units 1 dose units in rats n=4 / dose

39 ADHD abuse has been consistently trending upward and, unless preventive measures are taken, is likely to evolve into the next opioid abuse epidemic in the U.S. MARKET OPPORTUNITY: ADHD The Next Epidemic: ADHD Medication Abuse on the Rise The ADHD Market is Expected to Reach $25Bn by 2025 (4) The picture can't be displayed. Branded ADHD market is $6 billion The picture can't be displayed. Prevalence of ADHD patients has grown 10.2% per year since 2013 The picture can't be displayed. 53% of prescriptions for ADHD drugs are for adults The picture can't be displayed. 12% of all U.S. children are diagnosed with ADHD, of whom 2/3 receive pharmacotherapy • The $12.5 Bn ADHD market with prescription growth of >4% year - over - year (1) • 10.5 million adults have ADHD and are the largest part of the ADHD market comprising of 53% of total TRx (1,2) • ADHD is the most common neurodevelopmental disorder of childhood (3) • 5 Million adults misused stimulant medication annually (4)

40 • Ensysce’s TAAP TM and MPAR TM platforms can be easily integrated into ADHD prescription drugs by using the same TAAP platform the Company developed for opioids • Ensysce is currently in the development and testing phases for its ADHD drug, TAAP TM PF8026, and is seeing promising results • PF8026 has shown to provide a safer immediate release (IR) amphetamine with a reduced abuse potential • PF8026 has a powerful chemical barrier that makes it hard for a potential abuser to isolate the amphetamine by means of kitchen chemistry Ensysce is working towards technology to address this up - and - coming crisis before it expands to make an enormous negative impact on the welfare of U.S. citizens PREVENTING THE ADHD EPIDEMIC